H2[IrCl6]•6H2O 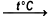 Ir + 2Cl2 + 2HCl + 6H2O
Ir + 2Cl2 + 2HCl + 6H2O
The thermal decomposition of hexahydrate hydrogen hexachloridoiridate(IV) to produce iridium, chlorine, hydrogen chloride and water. This reaction takes place at a temperature of over 700°C.