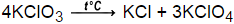2KClO3  2KCl + 3O2
2KCl + 3O2
The thermal decomposition of potassium chlorate to produce potassium chloride and oxygen. This reaction takes place at a temperature of 150-300°C. In this reaction, the catalyst is can be manganese(IV) oxide.
Other variants:
Other variants: