Also called the TriCarboxylic Acid cycle (TCA) or Krebs cycle, because it was he who suggested the existence of such a cycle. (1937)
https://en.wikipedia.org/wiki/Citric_acid_cycle
16 years later he was awarded the Nobel Prize for Physiology or Medicine. Then the discovery was something really important. What is this cycle and why it's so important?
We have to start from quite far away. Since you're reading this, you should already know about the main energy source for any cell of our body, which is glucose. Blood contains almost constant quantity of it, the latter being regulated by a special mechanism.
In each cell there are so-called mitichondria - separate organells ("organs" of a cell) which transform energy to produce a special intracellular energy carrier, called ATP (adenosintriphosphoric acid). It is widespread and very convenient energy source: it can attach directly to proteins, providing energy. The most simple example is myosin, a protein which makes muscle cells able to contract.
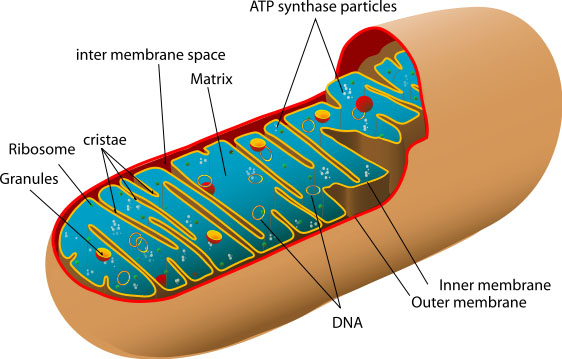
The picture of a human mitochondrion
Glucose cannot be transformed directly into ATP despite being very rich in energy. How to extract the energy from it without brutal methods like burning? There are roundabout routes which are possible thanks to enzymes (protein catalysts).
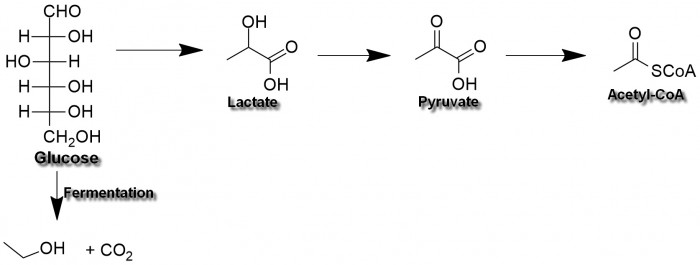
The first step is conversion of glucose into two molecules of pyruvate or lactate. It yields about 5% of all glucose's energy content. The lactate is formed in anaerobic conditions, i.e. when no oxygen is present. There is also a way to convert glucose into two molecules of ethanol and carbon dioxide, (called fermentation) but we won't consider this way now.
We won't scrutinise the mechanism of glycolysis (the conversion to pyruvate) either. Because, as it is written in Lehninger's book, "The conversion of glucose into pyruvate is mediated by ten enzymes working sequentially". If you really want to know more, have a look into any biochemistry textbook - the whole process has been extensively studied by specialists.
It seems that the conversion of pyruvate into carbon dioxide must be rather simple and straightforward. But it turned out to be a process of nine stages, which is called the Krebs cycle. This is a bit counterintuitive, but the cycle is used to interconnect different metabolic pathways - e.g. metabolism of aminoacids. Any compound which has to be utilized is either converted to energy or is used to build different compounds which the cell needs.
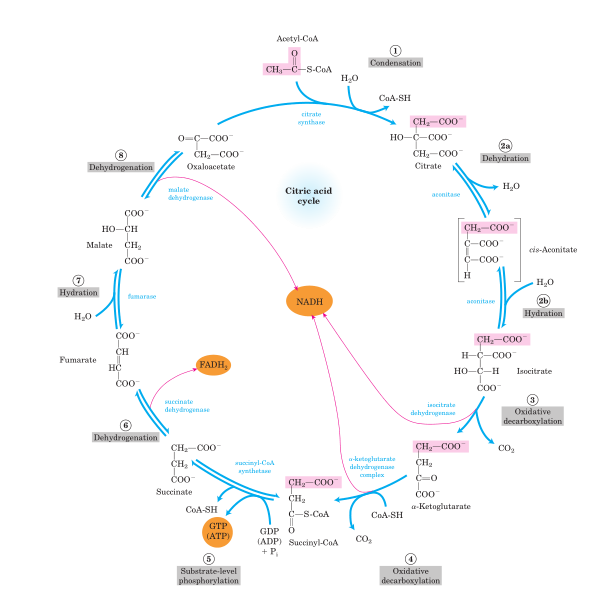
The whole Krebs Cycle
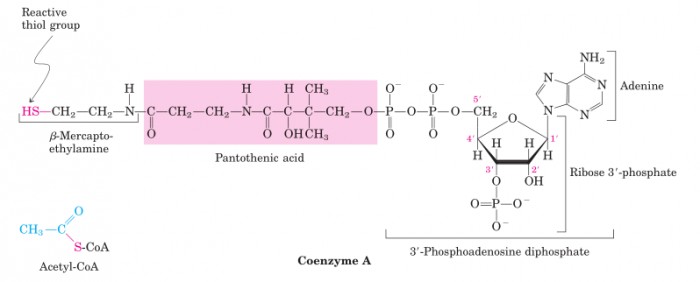
The first step is the oxidative decarboxylation of pyruvate into acetyl fragment (in the form of Acetyl-CoA). CoA is an coenzyme bearing a thiol group which can be used to transport and utilize acetyl fragments.
The structure of CoA
Lipids are metabolised into acetyl-CoA fragments too, which are directed to the Krebs cycle. Moreover, they are synthesized by the opposite (but somewhat similar) way from acetyl-CoA, which explains the fact that odd-numbered carbonic acids are very infrequent.
Acetyl-CoA and oxaloacetate are involved in condensation reaction yielding citrate, CoA-SH and a water molecule. This step is essentially irreversible.
Citrate is dehydrated to form cis-aconitate, the second tricarboxylic acid of the cycle.
Cis-aconitate adds a water molecule transforming itself into isocitric acid. This step is reversible.
Isocitric acid is decarboxylated and oxidized yielding alpha-ketoglutaric acid. Simultaneously NAD+ is reduced to NADH.
The next step is another oxidative decarboxylation. But what is formed is not succinate, but succinyl-CoA. It can then be hydrolyzed with release of energy in form of ATP molecule.
Then there are three reversible stages: dehydration of succinate into fumarate, addition of water to form malate and oxidation which leads once again to oxaloacetate. The circle is completed now, with one more molecule of NADH and FADH2 (another cofactor used in oxidative or reductive reactions) being produced.
It turns out that oxaloacetate functions as a catalyst, because it is neither produced nor consumed in the process. It really is the case: the concentration of oxaloacetate is maintained rather low in the mitochondria. But how to avoid the buildup of other products and how to synchronize all eight steps of the cycle?
It turns out that a special mechanism exist for this purpose. When there’s a buildup of any component, it blocks the enzyme which is responsible for its synthesis – an example of a negative feedback. It becomes even easier when reversible reactions are involved: reaction can just go in opposite direction. One may suggest that the cycle leads to considerable losses of energy, but it is not the case because the concentration of the products is strictly regulated in order to minimize the losses.