SnS2 + 16HNO3 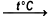 SnO2 + 2H2SO4 + 16NO2 + 6H2O
SnO2 + 2H2SO4 + 16NO2 + 6H2O
Tin(IV) sulfide react with nitric acid to produce oxide tin(IV), sulfuric acid, nitrogen dioxide and water. Nitric acid - concentrated solution. The reaction takes place in a boiling solution.