2Fe + 6H2SO4 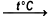 Fe2(SO4)3 + 3SO2 + 6H2O
Fe2(SO4)3 + 3SO2 + 6H2O
Iron react with sulfuric acid to produce iron sulfate (III), oxide sulfur (IV) and water. Sulfuric acid - concentrated solution. This reaction takes place heating the reaction mixture.
See also: