(NH4)2Cr2O7 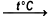 N2 + Cr2O3 + 4H2O
N2 + Cr2O3 + 4H2O
The thermal decomposition of ammonium dichromate to produce nitrogen, chromium(III) oxide and water. This reaction takes place at a temperature of 168-185°C.
Laboratory method receiving of nitrogen.
Laboratory method receiving of nitrogen.