2[Cr(H2O)3Cl3] 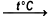 [Cr(H2O)4Cl2]Cl•2H2O + CrCl3
[Cr(H2O)4Cl2]Cl•2H2O + CrCl3
The decomposition of trichloridotriaquachromium(III) to produce dihydrate chloridotetraaquachromium(III) chloride and chromium(III) chloride. This reaction takes place at a temperature of 200-220°C.