2La(NO3)3•6H2O 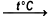 2LaONO3 + 4NO2 + O2 + 12H2O
2LaONO3 + 4NO2 + O2 + 12H2O
The thermal decomposition of lanthanum(III) nitrate hexahydrate to produce oxide-nitrate lanthanum(III), nitrogen dioxide, oxygen and water. This reaction takes place at a temperature of 600-780°C.